Abstract
The pharmacokinetic (PK) profile of factor IX (FIX) following an IV infusion is characterized by a normal incremental recovery (IR) followed by a rapid distribution phase with approximately 50-80% of injected FIX in patients disappearing within the first 5 min, and a slower terminal elimination phase. This is consistent with a two-compartment model where FIX largely distributes to a space outside of the plasma compartment (extravascular space). Mechanistically, extravascular distribution of FIX could potentially prime tissues for more efficient hemostasis following an injury. In people with hemophilia, this may be particularly important at sites prone to bleeding such as joints. With the introduction of extended half-life FIX products, it has become apparent that the type of modification to extend half-life can impact the PK profile, most notably the IR, and volume of distribution (Vss). For example, while rFIXFc shows an IR similar to rFIX and pdFIX and a larger Vss after an IV dose, modifications such as glycoPEGylation can lead to a higher IR and a smaller Vss indicating less distribution to tissue. The goal of this study is to evaluate the distribution of rFIXFc, rFIX and glycoPEGylated-rFIX (GP-rFIX) to tissue in hemophilia B mice using in vivo SPECT imaging.
GP-rFIX (sequence identical nonacog beta pegol, N9-GP) was generated following published methods applied to rFIX. rFIXFc was manufactured by Biogen and rFIX by Pfizer. rFIXFc, rFIX and GP-rFIX were labeled at lysine residues using an 125I-SIB (succinimidyl iodobenzoate) or 127I-SIB for "cold" experiments to assess impact of label (1:1 label:protein ratio) on activity and PK properties in hemophilia B mice. For SPECT imaging, hemophilia B mice were dosed with 125I-labeled rFIXFc, rFIX and GP-rFIX at therapeutically-relevant doses (10 nmol/kg, corresponding to ~50 IU/kg). Animals were imaged longitudinally on a NanoScan SPECT/CT (Mediso), and all animals were imaged individually (30 min SPECT scan followed by CT for anatomical reference) for a total of five time points. Maximum-intensity projection (MIP) images were generated and perfect injected dose per gram (%ID/g) determined for regions of interest (ROIs) using VivoQuant.
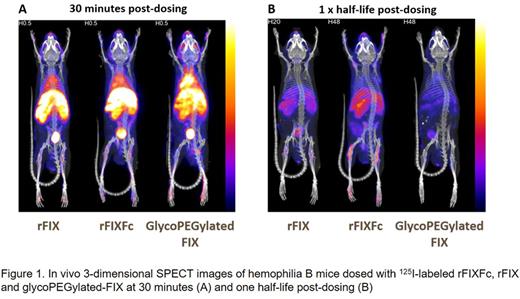
Labeling of the FIX variants with an 127I-SIB reagent did not impact the activity or PK properties of the molecules. In vivo SPECT imaging revealed higher amounts of GP-rFIX than rFIXFc or rFIX in highly vascularized tissues such as liver and blood pool at early time points (Figure 1), consistent with higher plasma recovery and a smaller Vss as reported for N9-GP. Both rFIXFc and rFIX showed higher distribution to joint areas such as knees and shoulders that persisted throughout all the time points measured (up to 5 half-lives post dosing), with rFIXFc showing significantly higher distribution to these areas, even at time points when FIX plasma levels were below the detection level. Interestingly, GP-rFIX showed minimal distribution to joint areas. Levels of rFIXFc and rFIX in the liver were greater than blood levels when normalized by weight, while the liver and blood levels were comparable for GP-rFIX, suggesting enhanced extravascular distribution and/or liver clearance for rFIXFc and rFIX compared to GP-rFIX.
The rFIXFc and rFIX biodistribution profiles in hemophilia B mice are consistent with the hypothesis that FIX can distribute to tissues outside of the plasma compartment. The significantly higher distribution of rFIXFc to joints areas compared to rFIX could potentially reflect retention at these tissues by an Fc-mediated mechanism or improved PK. These data are also consistent with the preclinical and clinical PK profile of N9-GP, and support the hypothesis that GP-rFIX remains mostly in the plasma compartment and shows reduced extravascular distribution due to modifications imposed by the PEG moiety. While the role of extravascular distribution in prevention of bleeding and overall protection of joint health remains an area of investigation, this study demonstrates biodistribution differences for FIX variants and illustrates plasma levels may not be comparable, or have the same meaning, across FIX variants. While SPECT imaging allows for the quantitative characterization of the biodistribution of FIX, the spatial resolution of the approach is limited. Ongoing studies are aimed at further characterizing the extravascular distribution of FIX with autoradiography and micro-autoradiography.
Salas: Bioverativ: Employment, Equity Ownership. Van Der Flier: Bioverativ: Employment, Equity Ownership. Hong: Bioverativ: Employment, Equity Ownership. Dumont: Bioverativ: Employment. Orcutt: inviCRO: Employment, Equity Ownership. Goel: inviCRO: Employment, Equity Ownership. Peters: Bioverativ: Employment, Other: Shareholder.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal